Share this Post
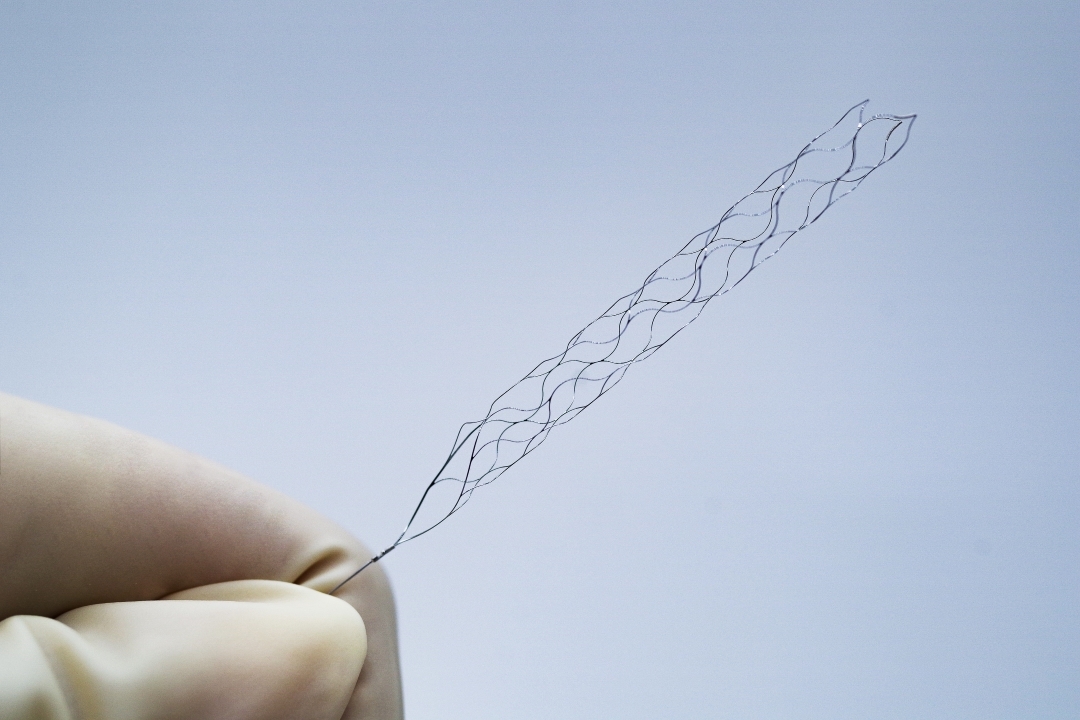
phenox Inc. a Wallaby Medical Company, announces the FDA 510k Clearance of the pRESET® Thrombectomy Device for the treatment of acute ischemic stroke.
The pRESET Thrombectomy Device is a minimally invasive device used to remove blood clots that cause acute ischemic strokes. The device is designed to be easy to use and can be deployed quickly, making it an ideal option for hospitals and clinics treating acute ischemic stroke patients. pRESET is indicated to reduce disability in patients with a persistent, proximal anterior circulation, large vessel occlusion, and smaller core infarcts who have first received thrombolytic therapy. phenox is only the 3rd company in the US to receive this device indication.
The acute ischemic stroke market is expected to grow significantly in the coming years, driven by an aging population and an increase in the number of procedures being performed. According to a recent report, the global acute ischemic stroke market is expected to reach more than $2 billion by 2025, with a CAGR of 5.12% during the forecast period.
"We are thrilled to be able to offer our pRESET Thrombectomy Device to physicians and their patients in the U.S.," said Andrew Cormack, Chief Commercial Officer at phenox Inc. "The pRESET device has been used successfully in Europe for over a decade, and we are confident that it will be well-received by US physicians as well. We believe that this device will play a critical role in the treatment of acute ischemic stroke, and we expect it to yield significant growth for our company."
phenox Inc. is committed to providing innovative medical devices to improve patient outcomes. The pRESET Thrombectomy Device is the latest addition to the company's portfolio of products available in the US, which includes a range of devices for the treatment of both hemorrhagic and ischemic stroke.
For more information about the pRESET Thrombectomy Device or to request a demonstration, please contact Phenox Inc. at cs@phenox.com
Note to editors: For more information, news, and perspectives from phenox Inc., please visit the phenox Inc. at (phenox.net) Web links, telephone numbers, and titles were correct at time of publication, but may have changed. For additional assistance, journalists and analysts may contact phenox Inc. corporate communications at cs@phenox.com
